High-energy density lithium-rich manganese-based layered oxide cathode material voltage for lithium ion batteries
The layered oxide cathode material (LiTMO2, TM is a 3d transition metal) is a key material for achieving high energy density lithium ion batteries above 300Wh / kg. Especially the lithium-rich manganese-based layered oxide cathode material (Li1 + xTM1-xO2, or can be written as xLi2MnO3 · (1-x) LiMO2) formed by the rock salt structural unit Li2MnO3 and the hexagonal layered structural unit LiTMO2, because it has twice The reversible lithium storage capacity of the first-generation lithium-ion battery cathode material LiCoO2 has attracted much attention (up to 300 mAh / g). However, the continuous voltage fade of this type of material during the electrochemical cycle has become the main bottleneck restricting its practical application. Due to the complex structure and chemical composition of lithium-rich manganese-based materials, complex charge compensation occurs in the electrochemical process and is accompanied by slow structural changes. The mechanism of voltage decay has been lacking in accurate understanding and conclusive experimental evidence.
Institute of Physics, Chinese Academy of Sciences / Beijing National Research Center for Condensed Matter Physics, Clean Energy Key Laboratory E01 Group, Yu Xiqian, and Brookhaven National Laboratory Ph.D. Enyuan Hu, Researchers Xiao-Qing Yang, Huolin Xin, Argonne, USA Laboratory researchers Jun Lu, Kahlil Amine and relevant researchers from the US National Institute of Standards and Technology have collaborated on advanced characterization methods such as in-situ X-ray absorption spectroscopy and three-dimensional transmission electron microscopy imaging to study carefully The voltage decay mechanism (Figure 1) clarifies the essential relationship between the instability of lattice oxygen ions participating in the redox reaction and the voltage decay and the effect of different elements on the voltage decay, and proposes corresponding solutions. The results of this study were recently published in Nature Energy (Nature Energy, 2018, 3, 690-698). The article is entitled Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release .
The research team used synchrotron radiation X-ray absorption spectroscopy combined with a specially designed simulated battery (Figure 2) to study in situ the lithium-rich manganese-based layered oxide cathode material for lithium-ion batteries (Li1.2Ni0.15Co0.1Mn0.55O2). The redox reaction mechanism of different charge and discharge cycles found that the transition metal cations Ni, Co, Mn and lattice oxygen anions simultaneously participated in the redox reaction, contributing to the lithium storage capacity and evolved with the electrochemical cycle (Figure 3). Among them, lattice oxygen ions participating in the reaction contribute a large amount of lithium storage capacity but are unstable, and Mn and Co gradually activate with electrochemical cycles to participate in the electrochemical reaction (reduced to cause voltage decay) and compensate for the capacity loss caused by the unstable oxygen participating in the reaction . The above research results clearly reveal the essential relationship between the reaction mechanism of lattice oxygen participating in the reaction to provide high capacity and the voltage decay of lithium-rich manganese-based materials. Furthermore, through the transmission electron microscope three-dimensional imaging technology, it was confirmed that the material gradually lost oxygen during the electrochemical cycle, and it was found that the reaction of the electrolyte with the electrode material will aggravate the material's oxygen loss and cause more serious voltage attenuation (Figure 4). This study indicates that suppressing the voltage decay of lithium-rich materials requires improving the stability of the lattice oxygen ions in the material during high-voltage charging, and different elements in the material have different effects on the voltage decay. This information provides ideas and experimental basis for the design of cathode materials for lithium-rich layered oxide lithium batteries with high capacity and stable structure without voltage attenuation. In addition, this is also the first experimental work to use in-situ real-time research on the evolution of the valence state and the performance attenuation mechanism of lithium-ion battery materials in the long-cycle process using synchrotron radiation experiment technology. This method and experimental design have important reference value for future research on the failure mechanism of batteries and battery materials in the long cycle life cycle.
Synchrotron radiation light source can provide a variety of non-destructive in-situ experimental techniques for studying the reaction mechanism of the electrochemical process of battery materials. Yu Xiqian and the research team of the E01 team of the Clean Energy Laboratory of the Institute of Physics have been committed to the development of battery research for many years. The in-situ experimental method has achieved a series of research results. Recently invited to write review articles in journals such as Chemical Reviews (2017, 117, 13123-13186) and Accounts of Chemical Research (2018, 51, 290-298) with international colleagues to introduce experimental methods of synchrotron radiation applied to battery materials research . Related work has been supported by the Ministry of Science and Technology's key research and development plan (2016YFA0202500), the Fund's Innovation Group Fund (51421002), the Chinese Academy of Sciences' Hundred Talents Program, and the China Youth Organization's Thousand Talents Program.
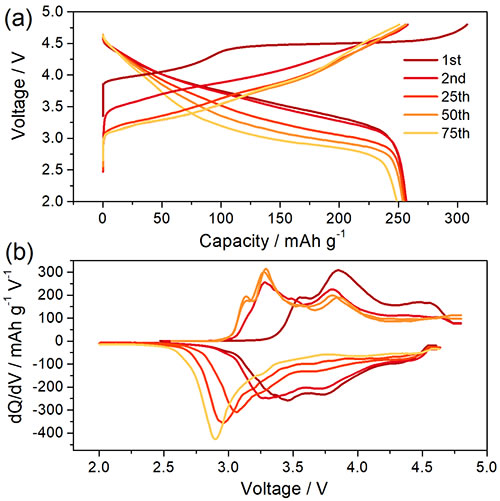
Figure 1 Li1.2Ni0.15Co0.1Mn0.55O2 (a) charge-discharge curve and (b) cyclic voltammetry curve at different charge and discharge cycles.
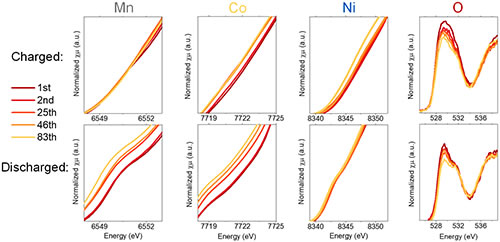
Figure 2 X-ray absorption spectra of different elements in Li1.2Ni0.15Co0.1Mn0.55O2 at different charge and discharge cycles.
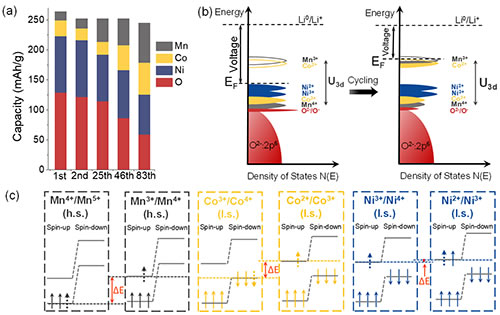
Fig. 3 Li1.2Ni0.15Co0.1Mn0.55O2 oxidation-reduction reactions at different charge and discharge cycles (a) the contribution of different elements to the capacity at different charge and discharge cycles; (b) the storage potential of lithium ions due to changes in electronic structure Change; (c) The energy level difference of redox electricity pairs of different transition metals is related to voltage decay.
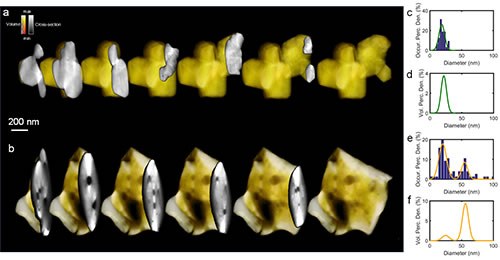
Figure 4 (a) and (b) three-dimensional morphology of the electrode particles before and after electrochemical cycling; (c) and (d) statistics of the internal pore size distribution of the material particles before cycling; (e) and (f) after cycling Statistics on the pore size distribution of internal micropores in material particles.
Bathroom Accessories,Bathroom Towel Holder,Bathroom Towel Storage,Bathroom Accessories Set
JIAHAOJIA SANITARY WARE INDUSTRY CO., LTD. , https://www.gagalfaucet.com