Blacktech Graphene Battery helps you stay away from "mileage anxiety"
Zhengdao Auto displayed the H600 model at this year's Shanghai Auto Show. The biggest highlight of the car is the power combination of "Micro-turbine generator ranger + super battery", which is arguably the most focused technology, its "micro-turbine generator The power combination of the range extender + super battery is in the category of extended range electric vehicles. The graphene super battery has an energy storage density of 300 watts per kilogram and the graphene battery can sustainably charge and discharge more than 40,000 times. The total combined mileage of oil and electricity exceeds 1,000 kilometers, and the acceleration time from 0 to 100km/h is only 2.9 seconds.
It is reported that the graphene lithium titanate battery is developed by Shanghai Dao Automobile and can realize rapid charging for 10 to 15 minutes. Compared with traditional new energy vehicles, it can improve the performance by 30% and the battery life can be as long as 10 years. In the case that the driving range has become the current "new taste" of new energy vehicles, the right way car can be said to be a problem for the current new energy vehicle charging and driving range problems, bringing a "big pot". However, these values ​​are limited to the concept car part, there is still a long period of time from the future production stage, whether the super battery can truly reach the driving range of more than 1,000 kilometers, but also need to speak through actual experience. The right road H600 has been unveiled at the previous Geneva Motor Show. During the Shanghai Auto Show, the right road car also brought two other concept models, the right road K550 and the right road K750.
From the current point of view, graphene is indeed much more advanced than current technology, but subject to cost and its own characteristics, it is now difficult to replace traditional lithium batteries in various fields. In general, the graphene lithium battery is still a laboratory product, and in the laboratory, there have been a series of cool methods such as nanotechnology to improve thermal stability and lifespan. Graphene is just one of them. . How the future of graphene batteries will develop will also need to be tested by the market and users.
Phase Transition Mechanism of Graphite Negative Electrode During Charge and Discharge
Modern lithium-ion batteries have been developed based on graphite anodes. During the charging process, Li+ desorbs from the anode lattice structure, diffuses through the electrolyte to the surface of the graphite anode, and then is embedded into the graphite structure. X-ray diffraction Neutron diffraction and other means have shown that as the amount of Li+ embedded in the graphite structure increases, LiC12 compounds are formed, the Li concentration continues to increase, and finally LiC6 compounds are formed. The entire process is divided into many steps, and current research shows that the LiC6 is completed. The embedded process can be divided into 4 stages or 8 stages.
Recently, MarTIn from Otto Schott Materials Research Institute in Germany used XRD technology and metallographic microscope technology to study the phase change of graphite negative electrode during lithium insertion and deintercalation. The study showed that the graphite negative electrode was inserted and deintercalated in lithium. There are different reaction mechanisms in the process, and LiC12 is a non-stoichiometric solid solution compound.
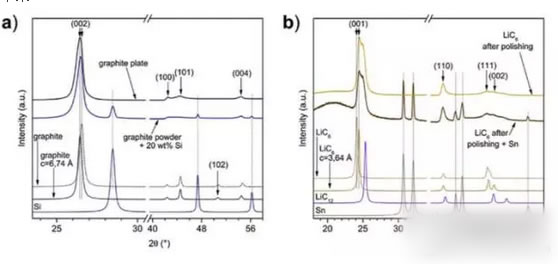
In the experiment, MarTIn graphite sheet (SGL Carbon Sigrafine 7500) and metal lithium powder were synthesized with golden LiC6 at 330°C. Other graphite samples with different lithium intercalation states were obtained by volatilizing LiC6 in vacuum and Li was volatilized. MarTIn also prepared a graphite sheet with a gradient Li concentration profile, as shown in Figure b below.
The XRD diffraction curve of the sample is shown in the figure below. Figure a shows the XRD pattern of the graphite flakes. As can be seen from the figure, the (002) diffraction peak moves to a low angle, indicating that the crystallographic parameters of the C axis have increased. The diffraction peaks of (100) and (101) broadened, while the (102) diffraction peak disappeared completely. This indicates that the graphite sheet is an disorderly chaotic arrangement. This may be the production process of graphite powder rolled into a graphite sheet. As a result, the XRD pattern of the LiC6 material shows that the disorderly chaotic arrangement in the graphite sheet is retained after lithium insertion.
The figure below shows the XRD patterns of the original, polished, and heat-treated LiC6 materials and graphite materials. From the figure, we can see that the original LiC6 material has a (001) diffraction peak at 24.5° and a wide diffraction peak. This may be due to poor crystallinity of the LiC6 material, or to a significant amount of LiC12.
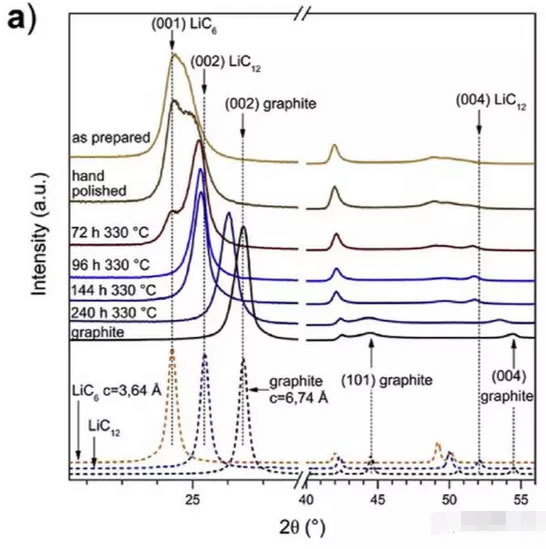
After polishing, the (002) diffraction peak of LiC12 appeared in the (001) diffraction peak, which may be due to slight changes in the structure due to the loss of Li during polishing. Different graphite intercalation materials can be obtained at 330°C for different time. After 72h treatment, the (002) diffraction peak of LiC12 is more obvious, and the (001) diffraction peak of LiC6 begins to decrease, indicating that the amount of Li decreases. The proportion of LiC12 began to increase, and the proportion of LiC6 began to decline. After 96 hours, the XRD pattern no longer contains the (001) diffraction peak, and the (002) diffraction peak position also shifts to a larger angle, indicating that the sample does not contain LiC6. After 146 h of treatment, the XRD pattern of the sample had almost no change compared to the sample heat treated for 96 h. After 240 h heat treatment, the (002) diffraction peak shifts significantly to a larger angle (29°), indicating that the c-axis lattice parameter of LiC12 is significantly reduced, the (101) diffraction peak and the (004) diffraction of graphite. The peak shows that Li in the sample is unevenly distributed in the graphite lattice.
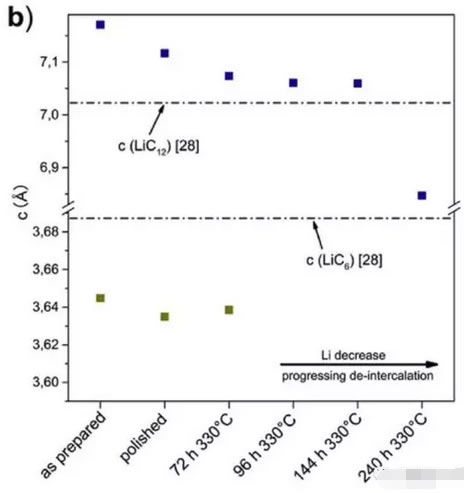
The figure below shows the c-axis lattice parameter variation data of different lithium-intercalated graphite samples. From the figure, it can be seen that the LiC6 component has very stable c-axis lattice parameters until decomposition disappears, and the c-axis lattice parameter of the LiC12 component is As the Li content decreases, the formation in the graphite structure is likely to be a solid solution structure.
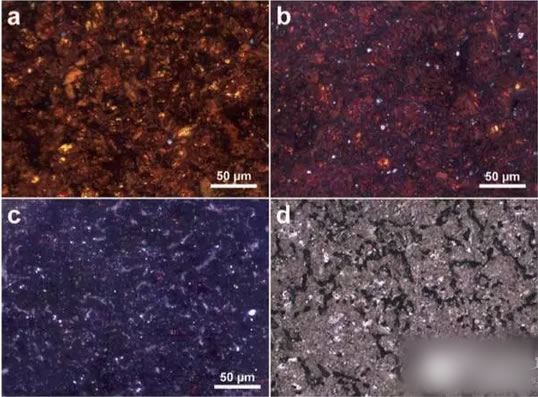
The picture below shows the metallographic micrographs of different lithium-intercalated graphites. Figure a shows the polished LiC6 sample. It can be seen that the sample contains 10-20um diameter gold particles (LiC6) with dark red phases around it. (LiC12). Figure b shows the samples heat-treated at 330 °C for 72 h. Compared with the samples without heat treatment, the samples had darker colors and more red areas. Figure c is a sample heat-treated at 330°C for 96 hours. It can be seen that there is only a small amount of red area in the image, and the main area has been changed to blue-violet. Figure d is the original phase structure of graphite. The entire picture area has only one phase and some micropores.

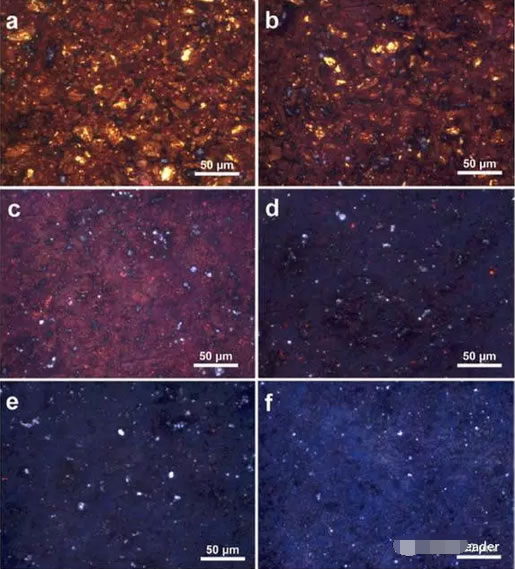
MarTIn performed a metallographic analysis on samples with gradient Li concentrations. The sample was divided into 7 regions as shown in the figure below. The metallographic image in Region 1 is shown in Figure a, which shows bright yellow areas and surrounding areas. In the red region, as the concentration of Li drops (Figures b, c, d), the yellow region gradually disappears, and the surrounding red region also turns into a blue-purple, which eventually turns into dark blue and gray (Fig. e, f).
The directly obtained XRD diffraction data of different Li concentration graphite samples and Li samples obtained after baking has a very significant difference, for example, in the samples obtained by calcination, the c axis cladding parameters of LiC6 components do not follow the Li content. The change of the change, and in the direct synthesis of Li gradient samples, as Li concentration decreases, the c-axis crystallographic parameters of LiC6 increase. In addition, the region 3 in the Li gradient sample (Fig. c) has the same composition as that obtained after the 72-hour baking, but the phase composition is obviously different. Both of the main phases are blue-violet, but the sample is obtained by baking. It still contains a considerable amount of LiC6 ingredients. This shows that Li has different reaction mechanisms during the insertion and extraction of graphite structures.
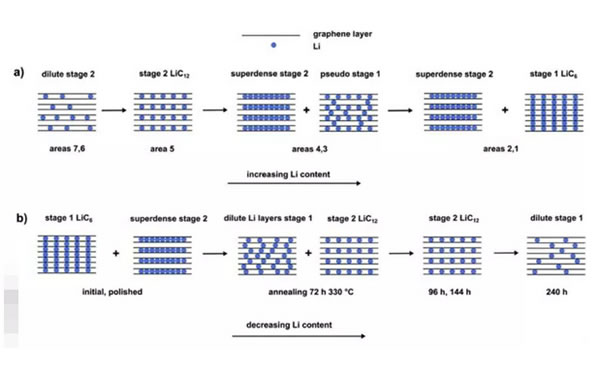
According to the experimental results and other research results, Martin Dru?e believes that the phase change of graphite in the process of lithium intercalation and delithiation is shown in the following figure. The lithium intercalation process (Fig. a) is first formed at a lower Li concentration. The low-concentration phase 2 phase starts to transform into ordered LiC12 as the amount of Li increases, and then the phase transitions to a mixed structure of high-density LiC12 and pseudo-phase 1 phase with the increase of Li, and finally into a super-density. LiC12 structure and LiC6 mixed structure.
In the process of Li out of the graphite structure (Fig. b), first the LiC6 phase and the super-density phase 2 phase transition to a low Li concentration layered phase 1 and ordered LiC12 mixed structure, and then with Li's further It is converted to an ordered LiC12, and eventually it is converted to a low-concentration Phase 1 phase as the Li concentration is further reduced.
Two types of phases were observed in samples of high Li LiC6 concentrations, one of which was bright yellow LiC6. One type of dark red LiC12 is quite different from our general cognition. Martin Dru?e believes that LiC12 is a kind of Li with different content, and its lattice structure can also have a continuous crystal structure transition in a very wide Li range, indicating that it is more like a solid solution structure. At the same time, the study also shows that Li has a different reaction mechanism when inserting and extracting graphite. During the process of embedding, a pseudo-phase 1 phase is formed. At the lower Li concentration, there is a larger lattice parameter. In the process of deintercalation, the lattice parameter of LiC6 hardly changes with the change of Li concentration, indicating that the stacking structure of Li and graphite is not changed, but a Phase 1 phase with low Li concentration is formed.
Bestware Stainless Steel Faucet brings the fine design and high technology together in all areas of the product process beyond Pull Out Faucet , Commercial Faucet and Commercial Kitchen Faucet. With extensive range of components, we can offer a large selection of both standard Pre-rinse Faucet and custom Basin Tap units as well as flexible combination. Stainless steel is 100% recyclable and is comprised of over 60% recycled material, Bestware faucets are the perfect solution in the commercial and industry for better water quality and the circumvention of the development of deleterious substances and bacteria. No plating, no oxidizing, no rust, lead free.
Stainless Steel Faucet,Black Stainless Steel Faucet,Stainless Faucet,Stainless Steel Bathroom Faucet
Bestware Hardware Production Co., Ltd. , https://www.bestwarefaucets.com